The plant made in one day 0.54 moles of oxygen.
From the balanced equation for photosynthesis, we know that 6 moles of oxygen are produced when 1 mole of glucose is produced at the same time.
So, to calculate the amount of oxygen that a plant made in one day when it produced 0.090 moles of glucose, we can use a mathematical Rule of Three:
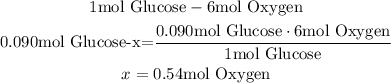
Finally, the plant made in one day 0.54 moles of oxygen.