Answer:
Step-by-step explanation:
Here, we want to get the pH of the buffer
To get this, we use the following equation:
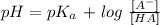
Where:
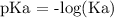
We have the A^- as the concentration of the salt and HA is that of the weak acid
Now, let us calculate the pKa as follows:
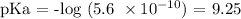
From Let us get the concentration of the weak base and that of the salt:
For ammonium sulphate, we have:
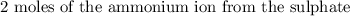
This means we have the concentration of the a