You can see that in the reaction 2 moles of NaOH reacted produce 2 moles of H2O, so the molar ratio between these two is 2:2 and simplifying it would be 1:1, this means that the moles of H2o will have the same number of moles of NaOH: 6 moles of NaOH will produce 6 moles of H2O.
The next step is to convert from 6 moles of water (H2O) to grams and we can do this using the molar mass of water which is 18 g/mol (you can calculate this value using the periodic table and doing the correct algebraic sum). The conversion would be:
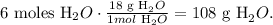
The answer is that we're going to obtain 108 g of H2O from 6 moles of NaOH.