The question requires us to provide the correct charge for a variety of ions, being lone elements or a combination of atoms.
For ions where there are more than one element, we need to analyze the charges of all atoms to achieve the total charge.
For example, for the ion NH4:
H presents charge +1
N presents charge -3
If we calculate the total charge, considering the number of atoms of each element, we have: 1*(-3) + 4*(+1) = +1
Therefore, the charge is +1 and the ion is written as:
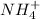
Next, for the ion NO3:
O usually presents charge -2
N might have a varying charge. In this case, it presents charge +5
The total charge is: 1*(+5) + 3*(-2) = -1
Therefore, the charge is -1 and the ion is written as:
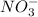
Next, for CO3:
O usually presents charge -2.
C presents charge +4 (just remember it normally makes 4 bonds)
The total charge is: 1*(+4) + 3*(-2) = -2
Therefore, the charge is -2 and this anion is written as:
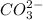
Next, for SO4:
O usually presents charge -2
S presents charge +6
The total charge is: 1*(+6) + 4*(-2) = -2
Therefore, the charge is -2 and this anion is written as:
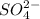
At last, for PO4:
O usually presents charge -2 and
P presents charge +6
The total charge is: 1*(+6) + 4*(-2) = -2
Therefore, the charge is -2 and this anion is written as:
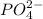
Now, for the ions made of just one element, we need to analyze the electron distribution.
For example, for Mg which has atomic number 12, the electrons are distributed as:
1s2 2s2 2p6 3s2
Thus, there are 2 valence electrons and it is easier to lose this electrons rather than gain 6 more (to achieve the octet). Since Mg can lose 2 electrons, it forms an anion with charge +2.
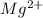
Next, let's write the electronic distribution for the elements Al, Na, K, Cl and O so we can make a similar analysis for them. (Note that, to make it easier, I'll be using the notation with noble gases)
Al: [Ne] 3s2 3p1
There are 3 valence electrons in Al - this atom loses 3 electrons and usually forms the ion Al3+
Na: [Ne] 3s1
There is 1 valence electron in Na - this atom loses this electron and forms the ion Na+
K: [Ar] 4s1
just like Na, there is 1 valence electron in K - this atom forms the ion K+
Cl: [Ne] 3s2 3p5
There are 7 valence electrons in Cl - in this case, it is easier to gain 1 electron to complete the octet - this atom forms the ion Cl-
O: 1s2 2s2 2p4
There are 6 valence electrons in O - in this case, it is easier to gain 2 more electrons to complete the octet - this atom usually forms the ion O2-
We can use a similar logic to find the charge for the ions formed by Cu and Fe. On the other side, these elements are transition metals and they can form ions with different charges.
Fe: 1s2 2s2 2p6 3s2 3p6 4s2 3d6
There are 2 valence electrons - this atom can lose 2 electrons and form the ion Fe2+, but it can also form the ion Fe3+
Cu: 1s2 2s2 2p6 3s2 3p6 4s1 3d10
Even though the electronic distribution shows us that the ion formed is Cu+, the most common ion for copper is Cu2+