We are given the following reaction:
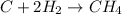
We are given that the number of moles of CH4 is 14.1 moles.
The question is asking for the mass of Hydrogen needed to produce 14.1 moles of CH4.
Solution:
First use the stoichiometry to find the number of moles of Hydrogen using the number of moles of CH4.
Two moles of hydrogen are required to produce one mole of CH4, therefore:
number of moles of H = number of moles of CH4 x 2
n of Hydrogen = 14.1 x 2
n of Hydrogen = 28.2 moles
Now we can use the number of moles to calculate the mass of Hydrogen.
n = m/M where m is the mass and M is the molar mass
m = nM
m = 28.2 x 1
m = 28 g