The equation for density is as follows:
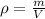
Where ρ is the density, m is the mass and V is the volume.
We want the unit to be g/cm³, so we can get these before putting the mass and volume into the equation.
The frame displaces 0.250 L of water, so this is its volume. To convert to cm³ we simply multiply it by 1000:
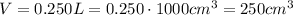
Its mass is 1.21 kg. To convert to g, we need to multiply it by 1000:
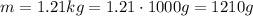
Putting these into the formula, we have the density:
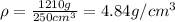
Thus, the density of titanium is 4.84g/cm3