The energy provided to the closed system is Q = 24059 J
The change in internal energy is
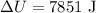
Let the work done be denoted by W
According to the first law of thermodynamics,
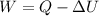
Substituting the given values, the work done will be
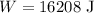
The work done will be 16208 J.