SOLUTION
Given the question in the image, the following are the solution steps to answer the question.
STEP 1: Write the given parameters
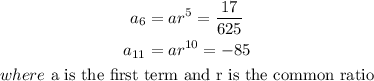
STEP 2: Divide the two expressions to get the common ratio
![\begin{gathered} (a_(11))/(a_6)=(ar^(10))/(ar^5)=-(85)/((17)/(625)) \\ a\text{ cancels a} \\ r^(10-5)=-85/(17)/(625) \\ r^5=-85*(625)/(17)=-5*625=-3125 \\ r=\sqrt[5]{-3125}=-5 \\ common-ratio=-5 \end{gathered}](https://img.qammunity.org/2023/formulas/mathematics/college/g2kwng8fbdchlqe6fdtwy3cv20o3j3ds0s.png)
STEP 3: Get the first term
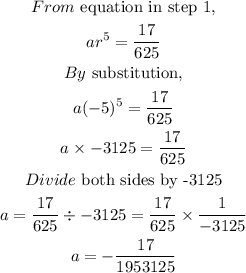
STEP 4: Calculate the 14th term
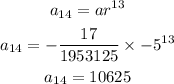
Hence, the 14th term is 10625