To solve this question we have to apply Charles Law, that states that:
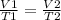
Where V1 is the initial volume, T1 is the initial temperature, V2 is the final volume and T2 is the final temperature.
Before using the given values, we have to convert the given temperatures to Kelvin degrees:
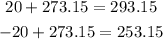
Now, we can replace these values for T1 and T2 respectively. Also replace V1 for 1L and solve for V2 to find the volume of the balloon inside the freezer:
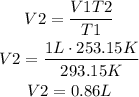
It means that the volume of the balloon in the freezer is 0.86L.