Answer:
96kPa. Option C is correct
Explanations:
According to Gay Lussac's law, the pressure of a given mass of gas is directly proportional to the temperature provided that the volume is constant. Mathematically:
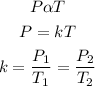
Substituting the given parameters to determine the new pressure P2
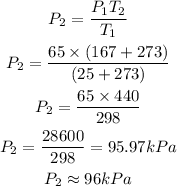
Therefore the new pressure is 96kPa