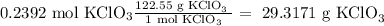Answer
29.3171 g KClO₃
Procedure
To solve this question consider the following chemical reaction:
2KClO₃→2KCl+3O₂
Then we will use the ideal gas formula to determine the number of moles present in 6.75 L of oxygen gas.
Data:
V=6.75 L
T= 298 °K
P= 1.3 atm
R= 0.08206 L⋅atm⋅°K⁻¹⋅mol⁻¹
Equation
PV=nRT
Solve for n to get the moles
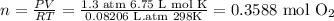
Use the stoichiometry of the reaction to convert from moles of oxygen to moles of KClO₃.
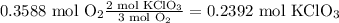
Finally, convert from moles to grams