ANSWER
Moles of water is 3.50 moles
Step-by-step explanation
Given that:
The mole of hydrogen gas is 3.50 moles
Firstly, write a balanced equation for the reaction
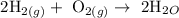
In the reaction, 2 moles of hydrogen will give you 2 moles of H2O
Since the number of moles of hydrogen given id 3.5 moles, then the number of moles of water.