First, let's convert gal to liters:
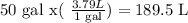
Let's assume this: as we work with oxygen gas, we consider STP conditions: 273.15 K (0 °C, 32 °F) and absolute pressure of exactly 1 atm (101.325 kPa). Nowadays we use 1 bar of pressure.
Another assumption is that oxygen gas is diluted as an O2 molecule.
Now we can do this:
At STP conditions for a gas:
1 mol of gas = 22.4 L = 6.022 x 10^23 formula units (formula units are molecules, atoms, etc.)
Procedure:
22.4 L of O2 -------- 6.022 x 10^23 molecules
189.5 L (50 gal) of O2 -------- x
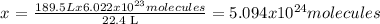
Answer: 5.094x10^24 molecules