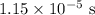Radioactive decay is characterized by the formula
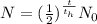
Where N is the amount of the element remaining,
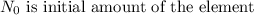
t is the time taken to reduce the amount of the element to N and th is the half life of the element
In this case
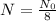
And
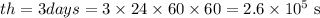
We need to find t
So we substitute the known values in the above equation
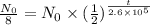
Which is
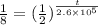
Taking the log on both sides,
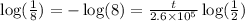
Simplifying,
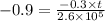
On rearranging and further simplifying we get,
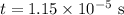
Thus the time taken for fermium 253 to reduce to one-eight of its initial amount is