2 ICl (g) <=====> I2 (g) + Cl2 (g)
Inicial: 0.682 g 0 0
(first we have only ICl, it doesn't react yet)
When reaction takes place: -2.x +x +x
(x is the amount that reacts,
e.g. -2 . x is the amount of ICl
that is consumed, for I2 and Cl2
we call +x because they are products)
Then in equilibrium:
First, we calculate molarity M
ICL = g/molecular mass . V(L) = 0.682 g/(162.35 g/mol . 0.625 L)=6.72x10^-3M
I2 = 0.0383 g / (253.80 g/mol . 0.625 L) = 2.41x10^-4 M
Remember everything is in the same volume
2 ICl (g) <=====> I2 (g) + Cl2 (g)
Inicial: 6.72x10^-3M 0 0
Reaction takes place: -2.x x x
Equilibrium: 6.72x10^-3M-2.x x x=2.41x10^-4M
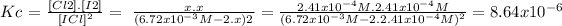
Kc = 8.64x10^-6