ANSWER
The mass of NH3 is 2.682 grams
Explanation
Given data
The number of NH3 molecules = 9.50 x 10^22 molecules
Let x represents the mass of NH3
Firstly, we need to find the number of moles of NH3 using the below formula
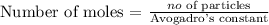
Recall that, Avogadro's constant = 6.02 x 10^23

From the above calculation, you will see that the number of moles of NH3 is 0.158 mole
The next process is to find the mass of NH3 using the below formula
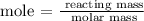
According to the periodic table, the molar mass of NH3 = 17 g/mol
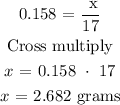
Therefore, the mass of NH3 is 2.682 grams