Answer:
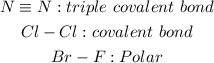
Explanation: Nitrogen triple bonding with nitrogen is a covalent bond. Each nitrogen needs three electron to fill its octet so both nitrogen atoms will share the electrons. Chlorine- Chlorine has a covalent bond. Each chlorine atom needs one electron to fill its octet and so both chlorine atoms share. Bromine-Flouride is polar because of the difference in electronegative, flourine being the more electronegative of the two.