ANSWER
The pressure of the gas is 3.9 atm
Step-by-step explanation
Given information
The volume of the gas = 5L
The temperature of the gas = 27 degrees Celcius
The number of moles = 0.8 moles
To find the number of moles, we will need to apply the below formula
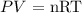
Where
P = pressure
V = volume
n = number of moles
R = gas constant
T = Temperature
Recall, that the gas constant is 0.082005 L atm mol^-1 K^-1
The next step is to convert the temperature from degrees Celcius to Kelvin
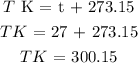
The next step is to substitute the given data into the formula in order to find the pressure of the gas
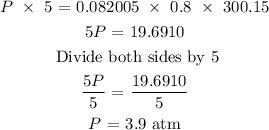
Hence, the pressure of the gas is 3.9 atm