Let's start by writing down the reactions of both solids solubilizing:
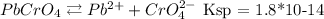
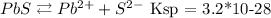
Now let's calculate the contribution of Lead (Pb) from each solid.
Starting with lead chromate. Since the compound ratio is 1:1, we can assume both ions will have the same concentration. So we can calculate using the Ksp constant and x for the unknown concentration:
Ksp = [Pb2+][CrO4 2-]
Ksp = x * x
Ksp = x²
1.8x10-14 = x²
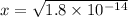
x = 1.3x10-7
[Pb2+] = 1.3x10-7 M (from lead chromate)
[CrO4 2-] = 1.3x10-7 M
Now let's do the same for lead sulfide:
Ksp = [Pb2+][S 2-]
Ksp = x * x
Ksp = x²
3.2x10-28 = x²
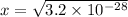
x = 1.8x10-14
[Pb2+] = 1.8x10-14 M (from lead sulfide)
[S 2-] = 1.8x10-14 M
Summarizing:
[Pb2+] = 1.3x10-7 M + 1.8x10-14 M
[Pb2+] = 1.3x10-7 M
[CrO4 2-] = 1.3x10-7 M
[S 2-] = 1.8x10-14 M