Given:
The parent nucleus is,
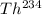
To find:
The product when Th-234 emits a beta particle
Step-by-step explanation:
When a particle emits a beta particle, the atomic number increases by 1, but the mass number remains the same.
So,
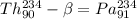
Hence, the product is Pa-234 with atomic number 91.