Answer
Given equation:
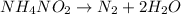
The oxidation number of the elements are assigned below:
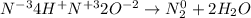
The oxidation number of N in NH₄⁺ is -3
The oxidation number of N in NO₂⁻ is +3
The oxidation number of N in N₂ is 0
The oxidation number of H is + 1
The oxidation number of O is -2
Oxidizing and reducing agents.
NH₄NO₂ is the oxidizing agent and is also the reducing agent.
Hence, the reaction is a comproportionation or synproportionation - a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number.
Change in oxidation number.
The oxidation number of N changes from -3 in NH₄⁺ to 0 in N₂
Also the oxidation number of N changes from +3 in NO₂⁻ to 0 in N₂