Answer:
2.007grams
Explanations:
The balanced reaction between aluminium and hydrochloric acid is given as:
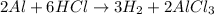
Determine the moles of Hydrogen gas
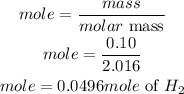
According to stoichiometry, 2 moles of Aluminium produces hydrogen 3 moles of hydrogen:
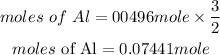
Determine the mass of Aluminium
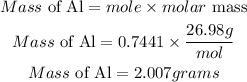
Hence the mass of Al required to produce 0.10grams of H2 is 2.007grams