Answer:
5.65moles
Explanations:
The formula for calculating the number of moles the compound contain is given as:
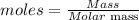
Given the following parameters
Mass of Ag = 700grams
Determine the molar mass of AgO
Molar mass = 107.87 + 16
Molar mass = 123.87g/mol
Determine the moles of AgO
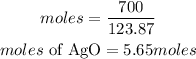
Hence the moles of AgO present is 5.65moles