Answer
The final volume of the solution be 2.13 liters
Step-by-step explanation
Given:
Molarity of the stock HNO3 solution, C₁ = 1.3 M
Volume of the stock HNO3 solution, V₁ = 0.6 L
Molarity of the dilute HNO3 solution, C₂ = 0.45 M
What to find:
The final volume of the solution (i.e the volume of the dilute HNO3 solution, V₂)
Step-by-step solution:
The final volume of the solution can be calculated using the dilution formula.
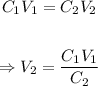
Plugging the values of the given parameters into the formula, we have;
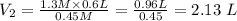
Therefore, the final volume of the solution be 2.13 liters