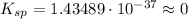So,
We could use the following reaction to find the Ksp value:
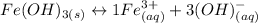
We know that the solubility of Fe(OH)3 is:
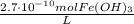
As you can notice, we could find the concentrations of each ion formed in the products using the stoichiometry of the reaction:
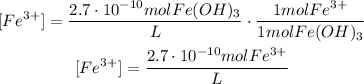
And now, we could do the same thing to find the concentration of the [OH]- ions:
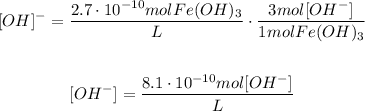
Now that we obtained each ion concentration, we could use the formula for Ksp:
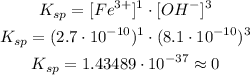
Thus, the ksp value for Fe(OH)3 given that that solubility is 2.7x10^-10mol/L is: