Answer:
B. T< 100 K
Step-by-step explanation:
1st) It is necessary to calculate the temperature with the given condition of ΔH and ΔS:
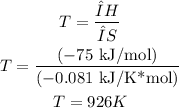
2nd) The reaction with negative enthalpy change and negative entropy change will be spontaneous only if the absolute value of the product between T and ΔS is less than ΔH:

At 926K the product will be equal to
So, at temperatures less than 100K the reaction will be spontaneous.