Step 1
The mass of I-131 left in the body could be calculated as follows:
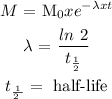
--------
Step 2
Data provided:
M = 5 μg (1 g = 1000000 μg) => 5 μg x (1 g/1000000 μg) = 5x10^-6 g
Mo = 5 g
Half-life = 8 hours = 8 h
-------
Step 3
Procedure:
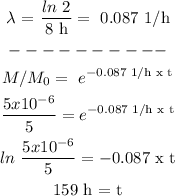
Answer: t = 159 h (approx.)