ANSWER
The amount of mole of NaOH used is 0.025 mol
Step-by-step explanation
Given that;
The starting amount of NaOH is 70mL
The ending amount of NaOH is 45mL
The molarity of NaOH is 1M
Follow the steps below to find the amount of NaOH used
Step 1; Write the molarity formula
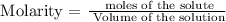
Step 2; Convert the volume to liters
1mL is equivalent to 0.001L
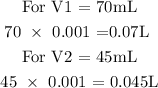
The amount of NaOH used at the starting point can be calculated below as

Therefore, the amount of NaOH used at the starting point is 0.07 mol
The amount of NaOH used at the ending point can be calculated below as

Step 3; Calculate the amount of NaOH used
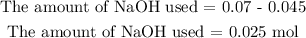
Therefore, the amount of mole of NaOH used is 0.025 mol