Answer:
Step-by-step explanation:
Here, we want to get the maximum amount of ammonia that can be produced
From the question, we have it that:
1 mole nitrogen gave 2 moles ammonia
Thus:
4 mole nitrogen will give 8 moles ammonia
Furthermore:
3 moles of hydrogen gave 2 moles of ammonia
5 moles of hydrogen will give:
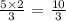
The maximum amount of ammonia that can be produced is thus 8.0 moles