The heat is given by:
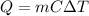
where m is the mass, C is the specific heat and delta T is the change in temperature.
In this case the mass is 225 g, this means that m=25.
The specific heat capactiy of water is 4.18 J/g°C then C=4.18.
Finally the change in temperature is

Plugging the values we have that:
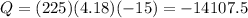
Therefore we need to remove -14107.5 J of heat. This is approximately -14.11 kJ