You can see that in the periodic table, the effective nuclear charge increases from left to right and from top to bottom as well.
So, we have to find which atom has the highest effective nuclear charge, watch the periodic table, and take into account the number of protons that contain. We can calculate the effective nuclear charge using the number of protons and the number of core electrons.
For P, we have that its electronic configuration is:
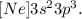
Summing the superindex for s and p (2 + 3 = 5), we're going to have that the effective nuclear charge is +5.
For Br, we have its electronic configuration:
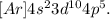
We have to do the same: summing the superindex for s and p (2 + 5 = 7), we don't take into account for d because it is a level of energy bigger and we can't have very high charges, so the effective nuclear charge is +7.
And finally, for Ba, the electronic configuration would be:
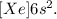
So, the effective nuclear charge is +2.
Remember that the smaller the charge, the greater the effective nuclear charge, so the answer would be Ba because it has a smaller nuclear charge.