1) List the known and unknown quantities.
Reactant 1: Acid
Sulfuric acid: H2SO4
Volume: 25.0 mL.
Molarity: unknown (mol/L).
Reactant 2: Base.
Ammonia: NH3.
Volume: 32.8 mL
Molarity: 0.116 mol/L
2) Balance the chemical equation.
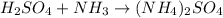
List the elements in the reactants.
H: 5
S: 1
O: 4
N: 1
List the elements in the products.
H: 8
S: 1
O: 4
N: 2
3) Balance N.
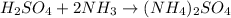
List the elements in the reactants.
H: 8
S: 1
O: 4
N: 2
List the elements in the products.
H: 8
S: 1
O: 4
N: 2
4) Find moles of ammonia (NH3)
Molarity: 0.116 mol/L.
Volume: 32.8 mL = 0.0328 L
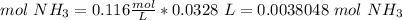
5) Convert moles of NH3 to moles of H2SO4.
The molar ratio between NH3 and H2SO4 is 2 mol NH3: 1 mol H2SO4.
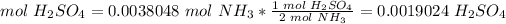
6) Molarity of H2SO4.
Moles: 0.0019024
Volume: 25.0 mL = 0.025 L
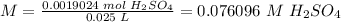
The concentration of H2SO4 is 0.08 mol/L.
Option D.
.