Answer:
The pressure of the sample is 1.18atm.
Step-by-step explanation:
1st) The given information from the exercise is:
- Number of moles (n): 1.24moles
- Volume (V): 24.5L
- Temperature (T): 284K
- Ideal gas constant: 0.0821 atm*L/mol*K
2nd) With the Ideal Gases formula, we can replace the values of n, V and T to calculate the pressure of the sample:
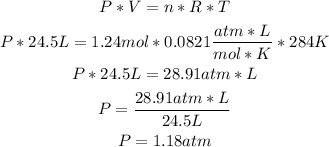
So, the pressure of the sample is 1.18atm.