Answer:
The density of the metal alloy is 7,496.25 kg/m^3 (rounded to 7,496 kg/m^3).
Step-by-step explanation:
The given information from the exercise is:
- Metal alloy mass (m): 100kg
- Mass of metal A (mA): 60kg
- Mass of metal B (mB): 40kg
- Density of metal A (dA): 9,000 kg/m^3
- Density of metal B (dB): 6,000 Kg/m^3
1st) With the values of mass and density of each metal, we have to calculate the volume of each metal:
• Volume metal A:
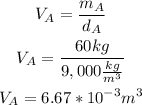
• Volume metal B:
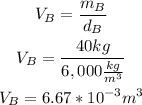
The volume of metal A and metal B is 6.67x10^-3 m^3. So, we have to add them to obtain the total volume of the metal alloy:
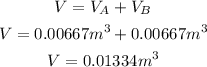
2nd) Now we can calculate the metal alloy density, replacing the values of Volume (V) and the metal alloy mass (100kg) in the density formula:
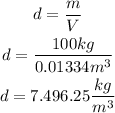
So, the density of the metal alloy is 7,496.25 kg/m^3 (rounded to 7,496 kg/m^3).