Answer:
23.58 years.
Explanation:
The decay equation for the radioactive element is:
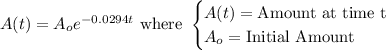
We want to find the half-life of the element.
The half-life of the radioactive substance is the time it will take for half of the initial amount of substance to decay. That is when:
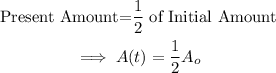
Substitute A(t) into the formula.
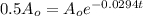
We then solve for t.
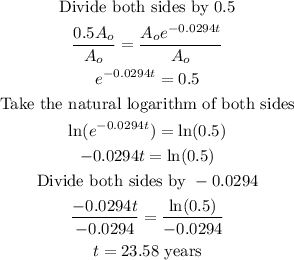
The half-life of the radioactive element is 23.58 years (correct to the nearest hundredth).