Answer:
The total energy of the reaction is 2,237kJ/mol.
The reaction is endothermic.
Step-by-step explanation:
To calculate the total energy of the reaction, it is necessary to subtract each bond energy in the reaction:
- Bonds broken (to the left of the arrow):
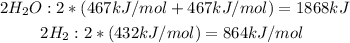
Total broken bonds: 2,732kJ/mol
- Bonds made (to the right of the arrow):
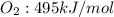
Total made bonds: 495kJ/mol
Finally, we have to subtract the total broken bonds minus total made bonds:
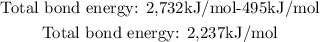
So, the total energy of the reaction is 2,237kJ/mol, and the positive sign means that the reaction is endothermic.