Answer:
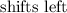
Step-by-step explanation:
Here, we want to know what will happen when reactants are removed from a chemical reaction in equilibrium
What happens here is that less of the product will be formed
That points to the fact that the equilibrium will shift to the left in favor of the reactant side