Answer
V2 = 10.4 L
Step-by-step explanation
Given:
Volume 1 = 12.4 L
Volume 2 = ?
Temperature 1 = 23°C = 296 K
Temperature 2 = 40°C = 313 K
Pressure 1 = 0.956 atm
Pressure 2 = 1.20 atm
Required: Volume 2
Solution:
The equation to solve this problem is the following:
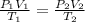
V2 = (P1 x V1 x T2)/(T1 x P2)
V2 = (0.956 x 12.4 x 313)/(296 x 1.20)
V2 = 10.4 L