The question requires us to calculate the concentration, in mol/L, of H3O+ and OH- ions for a 0.00016 M NaOH solution.
Since NaOH is a strong base, we can consider that it completely dissociates in water:
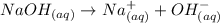
From the dissociation reaction shown above, we can say that the concentration of OH- ions in a NaOH solution will be the same as the concentration of NaOH.
Thus: [OH-] = 0.00016 mol/L
Now, we can apply the ion-product constant of liquid water (Kw) to calculate the concentration of H3O+ ions:
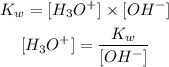
Since the value of [OH-] is 0.00016 M and considering the value of Kw as 1.00 x 10^-14, we calcuate [H3O+]:
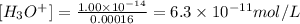
Therefore, the concentration of [OH-] and [H3O+] ions are 1.6 x 10^-4 and 6.3 x 10^-11 M, respectively.