Step 1 - Understanding why the volume is important
Kinetic molecular theory is interested in relating microscopic properties, such as molecules velocity, to macroscopic properties, such as temperature, volume and pressure.
Let's say we want to deal with the volume of a gas for some calculation sake. The volume of a gas could be defined as the sum of two volumes: the volume occupied by the molecules themselves and the volume between them:
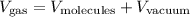
Now, considering that the molecules can be approximated by spheres, the volume of a sphere is:
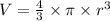
So we would get a fairly complicated expression for the volume of a gas, which would depend on the radius of the molecules (which would be quite hard to measure) as well as on the number of molecules (N):
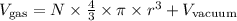
Step 2 - How do we deal with the volume of the molecules then?
Let's suppose we could calculate the volume of all N molecules. The matter of fact is that this number would be completely irrelevant to the total volume, because it would be too small compared to the volume between molecules.
We can make our calculations easier by assuming, thus, that:
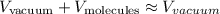
Therefore, the volume of the gas could just be described as:
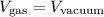
Which is a rather more simplified version of the equation. It simply states that the volume of the molecules is not relevant because it is too small.
Step 3 - Choosing the right alternative
As we have discussed, the main point of neglecting the volume of atoms or molecules is simplifying the formulas. We can only do this because their volume is irrelevant compared to the volume between volumes.
The correct alternative, therefore, is item b, compared to the space between gas particles, the volume of the particles themselves will be too small to affect the calculation.