ANSWER
The mole of argon atoms that is present in 17.2L of argon is 0.7679 mole
Explanation:
Given parameters
• The volume of argon at STP = 17.2L
,
• Let x represents the mole of argon at STP
Recall that, 1 mole of a gas is equivalent to 22.4L at STP
The next thing is to establish a proportion between the number of moles and volume of the given gas
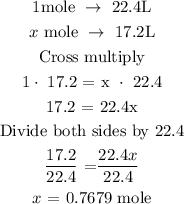
Hence, the mole of argon atoms that is present in 17.2L of argon is 0.7679 mole