Answer:
165.3915 grams
Explanations:
To get the amount of grams in 1.5moles of K2S, we will use the formula;
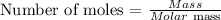
Given the following parameters;
Number of moles = 1.5 moles
Molar mass of K2S = 2(39.098) + 32.065
Molar mass of K2S = 78.196 + 32.065
Molar mass of K2S = 110.261g/mol
Get the mass of K2S;
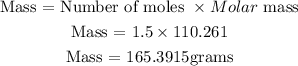
Hence the mass of 1.5 moles of K2S is 165.3915 grams