Question a
Step 1
The average atomic mass of the element is calculated as:
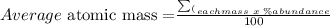
--------------------
Step 2
Information provided:
Isotope 1:
10.0 % abundant and 23.8 amu.
Isotope 2:
40.0 % abundant and 24.6 amu.
Isotope 3:
25.8 amu, the % abundant = 100 % - 10.0 % - 40.0 % = 50.0 %
-----------------------
Step 3
Procedure:
Average atom mass = (10.0% x 23.8 amu + 40.0% x 24.6 amu + 50.0 % x 25.8 amu)/100 = 25.12 amu
Answer: 25.12 amu