In order to determine the amount of radium after 800 years, use the following formula:
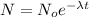
where,
N: amount of radium after t years = ?
No: initial amount of radium = 50g
λ: decay constant
t = 800
The decay constant is calculated by using the following expression:
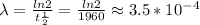
where t1/2 = 1960 is the half-life.
Now, by replacing λ, No and t = 800 you obtain:
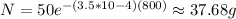
Hence, after 800 years there are approximately 37.68g of uranium