Step 1 - Understanding how to balance chemical equations
To balance a chemical equation, we must guarantee the number of atoms of the same type is the same in both sides of the equation.
To balance the equation, we can multiply the substances by some number, but we whould never, in any circunstance, modify the small numbers bellow the symbol of the elements.
That would drastically modify the substance that is participating in the reaction. Therefore, it would modify the reaction itself.
Let's take a look at an example:
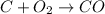
The number of C and O atoms is not the same in both sides. We could balance this equation like this:
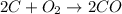
Now they're the same!
Step 2 - Balancing the given reaction
The given reaction is:
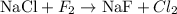
Let's start with Cl. Note there are two Cl atoms in the right-hand side (RHS), but only one Cl atom in the left-hand side (LHS). We can balance this by multiplying the substance NaCl by two:
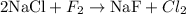
Now let's balance F atoms. Note there are two F atoms in the LHS, but only one F atom in the RHS. We can balance this by multiplying the substance NaF by two:
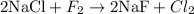
Now the equation is properly balanced. Note we have two Cl, two Na and two F atoms in both sides.
Step 3 - Naming the type of the reaction
Note that in this reaction the Cl that was bonded to Na exchanged places with the F. This is thus a single displacement reaction. In this reactions, one atom is "displaced" by another, which thus interacts with the same atom the displaced one was interacting before.