Let's start by identifying the compounds.
Ammonium sulfate has the ammonium cation (NH₄⁺) and the sulfate anion (SO₄²⁻). Thus the copound is: (NH₄)₂SO₄.
Calcium carbonate has calcium cation (Ca²⁺) and the carbonate anion (CO₃²⁻). So, the compound is: CaCO₃.
The reaction will be the exchange of the cations and anions:
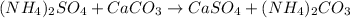
To form the calccium sulfate we want, CaSO₄.
The reaction is 1:1:1:1.
Let's now calculate how many moles we have of each reactant.
First, the molar weight of them:
Ammonium sulfate (NH₄)₂SO₄:
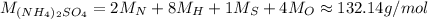
Calcium carbonate CaCO₃:
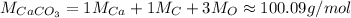
Now, the number of moles of each:
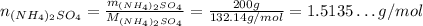
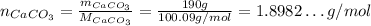
Since the reaction is 1:1 for the reactants and we have more (NH₄)₂SO₄ than CaCO₃, the (NH₄)₂SO₄ is the limitant. So, at most, approximately 1.