Answer:
Sum each order of each reactant in the reaction.
Step-by-step explanation:
To find the overall order of a reaction, it is necessary to sum each order of each reactant in the reaction (the order of the reactants it is not related to the coefficients).
For example:
- Reaction:
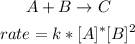
The reaction is first order in A and second order in B.
- Overall order:
Sum of the exponents: 1+2=3
The overall order for this reaction is 3.