Let's use the formula about density.
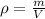
Let's use the given magnitudes. The density is 10.5 g/mL but we need to approximate it to the whole number, and the volume is 6.0 mL.
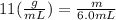
We have to multiply each side by 6.0 to find the mass.
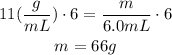
Therefore, the mass is 66 grams.