We will use the Ideal Gas Law to do this question:
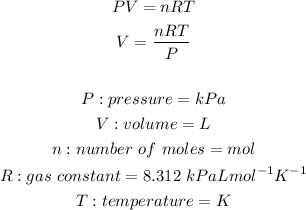
In order to move foward with the calculation, we must first do some conversions. We must convert the mass of oxygen gas into moles, we must covert the given units of temperature and pressure.
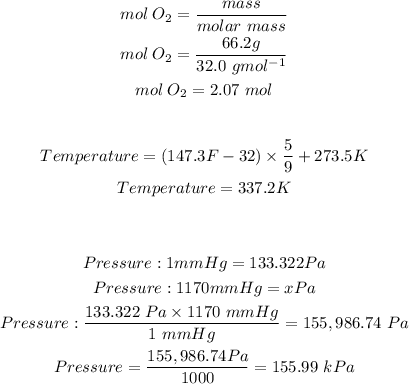
Substituting the values we have into the ideal gas law formula gives:
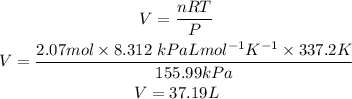
V= 37.19 L