The heat absorbed or released by a substance when its temperature changes depend on its heat capacity (Cp). The Cp of the water is 1 cal/g°C. The heat released can be found by the following equation:
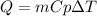
Where,
Q is the heat released or absorbed
m is the mas oof water, 15.5g
Cp is the heat capacity of water, 1cal/g°C
dT is the change in temperature
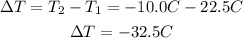
We replace the known values
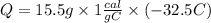
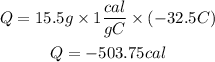
In Joules the heat released will be:
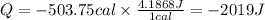
the heat has a negative value, this is because the water released heat.
Answer:
The amount of heat released is 504 calories or 2019 joules